
 Partnering for potential rollout of the first
Partnering for potential rollout of the first
HIV prevention ring
The dapivirine ring could add an important component to a future prevention toolkit as a long-acting and self-initiated option for women. Leveraging the partnerships that helped advance the ring through Phase III trials, IPM is collaborating across public, private and civil society sectors to increase widespread awareness, and to encourage demand and uptake if the ring is approved and incorporated into HIV prevention strategies.
self-initiated option for women. Leveraging the partnerships that helped advance the ring through Phase III trials, IPM is collaborating across public, private and civil society sectors to increase widespread awareness, and to encourage demand and uptake if the ring is approved and incorporated into HIV prevention strategies.
IPM works with both governmental and traditional leaders in South Africa and Uganda to ensure that discussions on national HIV prevention policies include the dapivirine ring as an important potential option for women. National strategic plans continue to support microbicide research and eventual introduction.
PARTNERING FOR ACCESS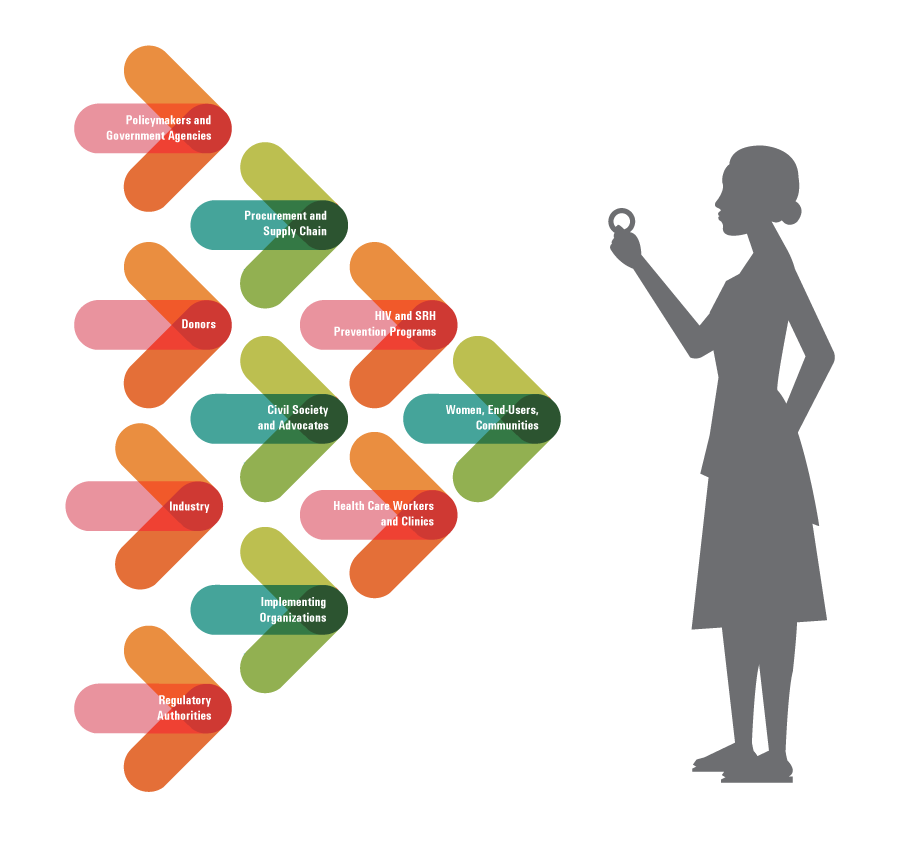
Activities in 2016 included:
- Purchasing the new inspection and packaging equipment needed to meet the manufacturing scale for the ring’s potential launch. IPM worked with its ring manufacturing partner, QPharma (Sweden), to install and prepare the equipment for process validation in 2017 to ensure the ring’s quality.
- Building a brand identity for the ring by conducting market research on potential brand names for the ring among health professionals in the US and Africa, and preparing for focus group discussions with potential end users in 2017. IPM will include the selected brand name in its MCC submission currently planned for late 2017.
- Exploring how human-centered design approaches could encourage dapivirine ring uptake and adherence among young African women, in partnership with Dalberg’s Design Impact Group and USAID’s Center for Accelerating Innovation and Impact.
- Partnering with USAID’s OPTIONS project to advocate for the ring’s inclusion in WHO guidelines and policies, and to develop an investment case for policymakers and global donors that demonstrates the ring’s potential public health impact and cost-effectiveness.
IPM expanded its partnership with Janssen Sciences Ireland UC, one of the Johnson & Johnson Pharmaceutical Companies, which granted IPM exclusive worldwide rights to dapivirine. Janssen committed to a multi-year secondment of a staff member whose commercial marketing expertise will help guide IPM’s market introduction activities for the dapivirine ring.
“Innovation can help rewrite the script for girls
and young women affected by HIV.”
– Jaak Peeters, global head, Global Public Health, Johnson & Johnson
< PREVIOUS MILESTONE | NEXT MILESTONE >
Courtesy of
3P Innovation Ltd.