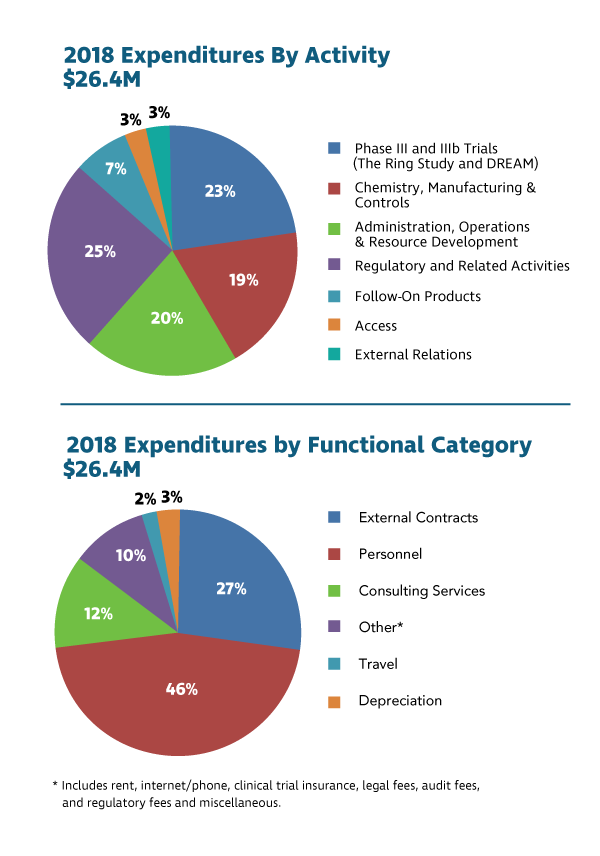
2018 Financial Considerations
IPM’s cash, cash equivalents and short-term investments as of Dec. 31, 2018, totaled USD 17.5 million. In 2018, IPM advanced four major programs:
- Supporting clinical research center partners and leading clinical activities for DREAM, an open-label extension study that provided the monthly dapivirine ring to former Ring Study participants for one year. The study began in July 2016 and ended in January 2019.
- Continuing chemistry, manufacturing and control activities to meet the scale needed for the ring’s launch, pending regulatory approval, and to support the ongoing EMA regulatory review and upcoming FDA submission.
- Conducting regulatory activities to seek approval for the dapivirine ring’s use in African countries where women face high HIV risk. IPM’s application to the EMA is currently under regulatory review, with an opinion expected in 2020. IPM also continued our work to prepare applications to SAHPRA and the FDA to be submitted in 2020.
- Advancing follow-on products that could help meet women’s HIV prevention needs at different times in their lives. The first clinical trial of a three-month dapivirine ring continued in 2018, with results expected in 2019. In addition, the first clinical trial of a three-month dapivirine-contraceptive ring completed in 2018, and a second Phase I trial began later in the year.
In 2018, IPM received support from donors including the Ministry of Foreign Affairs of Denmark, the Flanders Department of Foreign Affairs, the German Federal Ministry of Education and Research, Irish Aid, the Ministry of Foreign Affairs of the Netherlands, DFID, USAID and the Bill & Melinda Gates Foundation. IPM received approximately USD 32.9 million (cash receipts) in 2018.
IPM’s 2018 financial audits continue a history of full compliance with all financial reporting requirements from all US and international government and private donors. In 2018, IPM again received an unqualified, or clean, audit opinion across its South Africa, US and Belgium offices.
IPM’s Board of Directors, management team and staff remain dedicated to efficiently and effectively delivering on our mission to develop HIV prevention and other sexual and reproductive health technologies for women, and to make them available and accessible where they are urgently needed. Given the persistently high rates of new HIV infections among women and girls, especially in sub-Saharan Africa, woman-centered technologies like the long-acting dapivirine ring could help fill an important gap in the existing HIV prevention portfolio. Continued financial support for IPM’s work is essential to realizing the promise of microbicides for women’s sexual and reproductive health, and we continue to advocate for increased funds from existing donors and to pursue new funding sources.