Establishing Large-Scale Manufacturing Capacity
IPM selected QPharma, a contract developer and pharmaceuticals manufacturer with more than 35 years manufacturing experience based in Malmö, Sweden, to produce the thousands of dapivirine vaginal rings needed for the two Phase III clinical trials of the ring conducted from 2012-2016 and several smaller safety studies that comprised IPM’s Dapivirine Ring Licensure Program.
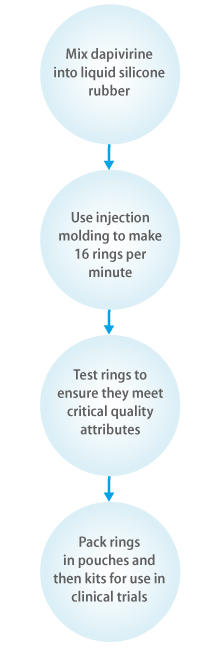
Highly specialized equipment is needed to manufacture the dapivirine ring. Because this machinery is not readily available, IPM purchased it for our manufacturing partner.
In 2014, QPharma completed the ring production for the licensure program.
Selecting a Manufacturing Partner for Scale-Up
In 2016, following Phase III results that showed the ring helps reduce women's HIV risk, IPM selected QPharma as its initial launch partner to manufacture the rings for open-label extension studies for former participants of the two Phase III trials.
To keep costs to women as low as possible, IPM has worked with QPharma (now Sever Pharma Solutions) to upgrade its manufacturing capacity to enable higher yields, including increasing automation and adding equipment that could simultaneously produce multiple batches of rings.
Collaborating to Meet Women’s Needs
IPM’s partnering approach to manufacturing offers a strategic and efficient way to ensure a stable supply of high-quality rings, and to minimize the time between potential approval and access to the product by the women who need it most.