Partnering for Potential Access: Dapivirine Ring
IPM’s most advanced microbicide, the monthly dapivirine ring, received a positive opinion from the European Medicines Agency in 2020 and a recommendation from the World Health Organization (WHO) in 2021. The product has also received WHO prequalification, a designation that confirms that it meets global standards for quality, safety and efficacy, and will help guide national and global procurement decisions, pending country regulatory approvals for its use.
IPM is prioritizing potential rollout of the ring in eastern and southern Africa, where women’s need for new HIV prevention tools is most urgent. IPM has submitted applications to Botswana, Kenya, Malawi, Namibia, Rwanda, South Africa, Tanzania, Uganda, Zambia and Zimbabwe. Regulatory approval was received in Zimbabwe and several other countries in 2021, with additional reviews underway. IPM will also seek to make the product available in Eswatini and Lesotho through national import license processes, and plans to submit to additional countries in Africa in the future.
Preparations for the ring’s potential rollout are also continuing. Market introduction efforts for other health and medical products have shown the need to meaningfully engage key stakeholders in order to minimize access delays.
IPM is applying these lessons and partnering with Johnson & Johnson, which granted IPM exclusive worldwide rights to dapivirine, to advance a comprehensive access strategy for the ring. Johnson & Johnson is lending its technical expertise and resources as IPM prioritizes and prepares for key access activities, including understanding end-user needs, demand forecasting and engaging healthcare providers.
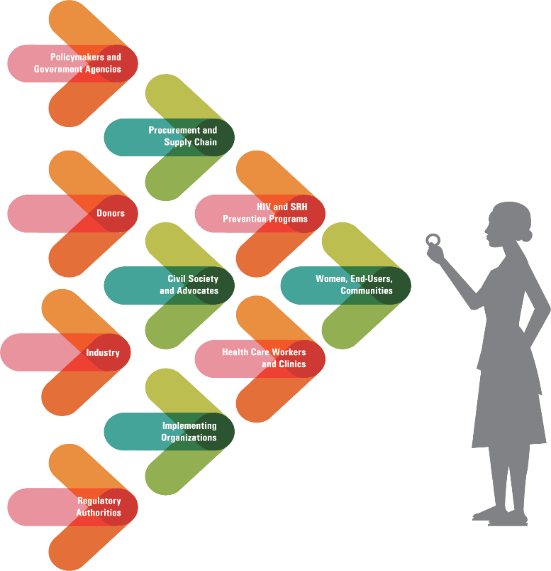
In addition to the partnerships that helped develop the ring, IPM is working with partners such as the USAID-funded PROMISE consortium and MOSAIC project across countries and sectors to prepare for the ring’s potential rollout. Activities include:
- Conducting stakeholder mapping and outreach to ensure key stakeholder involvement in all aspects of product delivery;
- Building investment scenarios and market-shaping strategies;
- Conducting market research, economic and public health modelling, and strategic product introduction research to address knowledge gaps and inform national policymaking and program implementation;
- Implementing a robust manufacturing strategy to ensure consistently high-quality products;
- Developing a supply chain and quality management strategy;
- Developing information, education and communications materials to increase awareness and build demand among women and their partners, communities and health providers; and
- Identifying financing mechanisms to ensure the ring is provided to women for free or at low cost.
IPM also established an Access Advisory Committee to provide strategic guidance to help ensure the ring’s successful introduction and uptake in sub-Saharan Africa, where women face the greatest risk for HIV.
Learn about IPM’s early microbicide access collaborations with the WHO and global stakeholders.