Dear friends and colleagues,
In 2002, a small but dedicated group of microbicide researchers and advocates helped form the International Partnership for Microbicides (IPM) to address what was then an already urgent need — new products women could control to help them stay free of HIV. Fourteen years later, by forging partnerships with public, private and civil society sectors to develop microbicides and building on lessons learned in the field, our collective efforts have begun to pay off.
Earlier this year, we announced that two Phase III clinical trials showed IPM’s monthly dapivirine ring can safely help reduce a woman’s risk of HIV. The road to developing an effective microbicide for women has been a long one — one that could not have been navigated without the contributions of researchers whose previous work informed the microbicide field; the inspiring commitment of the women who volunteered for clinical trials, and the communities and advocates who supported them; the researchers who developed and tested the ring; the pharmaceutical company that donated the drug; and the donors who invested in the ring’s promise.
The results also offer hope for a new generation of prevention options for women. IPM is advancing its pipeline of long-acting, self-initiated products, including a 90-day dapivirine ring, a 90-day dapivirine-contraceptive ring that would simultaneously prevent HIV and unintended pregnancy, and combination products that would leverage the potency of multiple drugs to prevent HIV.
This is an important moment in HIV prevention and women’s health, but much more needs to be done to achieve regulatory approval for the dapivirine ring and to deliver it to women. The field also has much to learn about how to best support women to use HIV prevention methods in general and to ensure new methods meet young women’s needs.
For over a decade, IPM has been guided by the conviction that, one day, women will have multiple HIV prevention options that work for them — from oral PrEP to rings and the host of other methods now in development. If we seize this moment to invest in products like the dapivirine ring, we could soon usher in that bright future for women everywhere.
Dr. Zeda F. Rosenberg Dr. James McIntyre
Although much progress has been made against HIV/AIDS, the rate of new infections among women remains alarmingly high and poses a crisis for women’s sexual and reproductive health. According to UNAIDS, the drop in the number of new adult infections has stalled and is in danger of reversing — and young women and girls continue to be at higher risk. This is especially true in sub-Saharan Africa, where nearly two of every three new infections in young people ages 15-24 occur in young women and one in four pregnancy-related deaths can be linked to HIV/AIDS.
New developments in HIV prevention research offer hope that women could soon have multiple self-initiated options they can use to protect their own health. Stopping the virus’s spread will require a range of prevention options that women can control themselves, because no single product will fit into all women’s lives.


Dapivirine Ring: A Long-Acting Tool for Women
The dapivirine ring developed by IPM marks a turning point in women’s health: The ring is the first long-acting HIV prevention product shown to safely help reduce infection and is the first microbicide to show efficacy in two Phase III trials.
IPM designed the flexible silicone ring to slowly release an antiretroviral drug (ARV) called dapivirine into the vagina over the course of a month. Women can insert and replace the ring themselves monthly.

The studies
IPM’s monthly dapivirine ring was evaluated in two Phase III clinical trials: The Ring Study, conducted by IPM, and ASPIRE, conducted by IPM’s clinical trial partner, the US National Institutes of Health-funded Microbicide Trials Network (MTN). The “sister studies” began in 2012 and enrolled over 4,500 participants in Malawi, South Africa, Uganda and Zimbabwe.
The results
IPM and MTN jointly announced results for both studies in February 2016 at the Conference on Retroviruses and Opportunistic Infections in Boston. Overall, the monthly ring reduced the risk of HIV infection among women ages 18-45 by approximately 30 percent, protecting about one in three women from infection. Less protection was seen among women ages 18-21. In ASPIRE, the ring cut HIV risk by more than half among women over age 21, who were able to use the product more consistently. Data from both studies strongly suggest that greater protection can be achieved with more consistent ring use, or adherence. Additional exploratory findings from ASPIRE announced in July 2016 suggest the ring may cut HIV risk significantly with consistent use — by at least 56 percent and potentially higher with near-perfect use. Full analysis of Ring Study data is forthcoming.








A soccer game in KwaZulu-Natal helped engage support for trial participants. Nearly 25 sporting events were held in Ring Study trial communities in 2015, reaching approximately 5,000 community members.
A community event in Masiphumelele helped raise awareness of the ring.
IPM staff at a community cookout in Limpopo.
Staff from clinical research centers participating in The Ring Study discuss adherence support strategies and prepare to share the
dapivirine ring efficacy results with trial communities.
IPM convened its clinical research partners to maintain the focus on adherence in The Ring Study.
2 - 5
<
>
Community outreach
Conducting HIV prevention studies requires extensive community outreach, involvement and support. IPM worked with community members in supporting The Ring Study and its participants through dialogues, small group discussions, events to encourage male engagement, and informational sessions and consultations to provide updates, address questions and build support in preparation for the dapivirine ring results announcement.


Next steps for the ring
IPM is collaborating with a network of public, private, research and civil society partners at global, national and community levels to realize the ring’s potential.
- Open-label studies
Two “open-label” studies, DREAM and HOPE, began in July 2016 to provide the dapivirine ring to former Ring Study and ASPIRE participants, respectively, and help show whether ring use will increase now that the product’s safety and efficacy are known. Researchers are encouraged by similar open-label studies of the daily oral ARV pill Truvada® used as pre-exposure prophylaxis (PrEP), which showed increases in adherence and efficacy once participants understood that the product worked to prevent HIV. Another study to begin in early 2017 seeks to better understand the prevention needs of adolescent girls and young women by assessing their preferences for and adherence to the monthly dapivirine ring or Truvada as daily PrEP. The study will also evaluate the products’ safety among this young high-risk population and will examine whether biological factors influence how the active drugs are taken up in the body. Together, these studies will also provide insights into adherence challenges and help identify new approaches to addressing them.
- Applications to regulatory authorities for the ring’s public use
IPM is assembling a comprehensive dossier of all data collected on the ring, as required by regulators to apply for product approval. Data analyses and compilation of the most recent study results are ongoing, with the first dossier submissions planned for mid-2017. The earliest potential product approvals could be received in late 2018.
Data from 14 smaller supporting studies will also be incorporated into the dapivirine ring regulatory dossier, including studies to assess ring safety in post-menopausal women, during menses and with tampon use, and with male or female condom use, as well as drug-drug interaction studies. - Access planning
IPM is working with governments, donors, communities and other partners across sectors and regions to determine how the dapivirine ring could best fit into HIV prevention programs and to accelerate affordable access if it is approved for public use. Activities include demand-forecasting and assessments of potential brand names, as well as the development of a market launch and access plan that identifies key financing, distribution and marketing strategies and activities. Initial stakeholder mapping exercises of four African countries identified possible manufacturing, distribution, marketing, civil society and other partners for the ring’s potential rollout. IPM is also collaborating with implementing partners of USAID’s Microbicide Product Introduction Initiative to ensure that the dapivirine ring is integrated into research and initiatives.


Left: A lab worker at the Madibeng Centre for Research in Brits processes a participant sample for The Ring Study.
Building Political and Public Support for Microbicides
From small meetings to a global stage, IPM engages civil society, policymakers and other stakeholders to focus
attention on the importance of advancing promising new HIV prevention technologies for women. In 2015, IPM worked closely with the global advocacy community to ensure that the UN Sustainable Development Goals (SDGs) included commitments to ending HIV/AIDS, investing in new HIV prevention technologies, and ensuring women’s sexual and reproductive health and rights. These efforts culminated in an IPM-hosted event ahead of the UN Sustainable Development Summit in New York that convened global development and health leaders to discuss the importance of innovation to achieve the SDGs.
IPM also partnered with civil society organizations to host the biennial South African National AIDS Council’s Women’s HIV Prevention Summit, and organized advocacy events and stakeholder briefings throughout the year across Africa, Europe, the United States and Canada to provide updates on the dapivirine ring studies and engage the advocacy community in advance of dapivirine ring Phase III trial results. IPM, MTN and the advocacy organization AVAC held joint consultations with civil society, government and health workers in the four clinical trial countries to share updates and request input on the design of the dapivirine ring open-label studies. This critical advocacy work will continue in 2016 as the ring moves toward potential regulatory approval and access.


Above: A community event at Ndlovu Medical Centre in Limpopo.
IPM’s Dapivirine Ring: A Product of Partnership
HIV/AIDS is the #1 cause of death among women ages 15-44 worldwide, and, in sub-Saharan Africa, young women ages 15-24 accounted for one in four new adult infections in 2015. Women need practical new tools they can use to protect themselves against HIV.

Partnerships were critical to the ring’s development:
THE WAY FORWARD:
The dapivirine ring offers new hope for expanding women’s HIV prevention options. Only through continued partnership can we realize the ring’s potential.
Next-Generation HIV Prevention for Women
IPM is leveraging its ring technology as a platform for new types of prevention products that may offer greater efficacy, more convenience and different options to meet women’s needs, which can change throughout their lives. A diverse pipeline that includes new ARVs is also critical to stay ahead of the evolving virus in the long term. Continued investment to develop a diverse pipeline of self-initiated products is essential to turning new hope into a
new reality.
3-month dapivirine ring
IPM is developing a ring that could be used for up to three months at a time, improving its convenience, lowering its yearly cost and reducing the frequency with which women would visit a health facility to obtain a new ring. This longer-acting dapivirine ring will be studied in the Phase I trial of the dapivirine-contraceptive ring (see next section), results from which will inform further development of both products.
3-month dapivirine-contraceptive ring
Two of the greatest risks to women’s health are HIV and unintended pregnancy, a leading contributor to maternal
mortality that is magnified in women with HIV. Women’s sexual and reproductive health needs do not exist in isolation, and
neither should their prevention choices.
IPM’s 90-day dapivirine-contraceptive ring is designed to slowly release dapivirine and the contraceptive hormone levonorgestrel to prevent both HIV and unintended pregnancy. In 2015, IPM produced the rings that will be studied in a Phase I trial of the multipurpose product in early 2017. In collaboration with MTN, this first-in-human trial will assess the safety as well as the pharmacokinetics (how the body processes the drugs) of the dapivirine-levonorgestrel ring and the three-month dapivirine-only ring.

DS003 products
IPM is pioneering the development of microbicides containing DS003, a gp120 entry inhibitor licensed from Bristol-Myers Squibb in 2005 that targets HIV early in its life cycle. In 2015, IPM initiated the first clinical trial of this novel ARV: a Phase I safety and tolerability study of a DS003 vaginal tablet. The results of this trial are expected in late 2016 and will inform the development of a vaginal ring that combines DS003 with dapivirine.
Rings with multiple ARVs
Combination rings that contain multiple ARVs with different mechanisms of action may offer greater protection against HIV and reduce the chance of acquiring drug-resistant virus. IPM pioneered the first combination microbicide tested in clinical trials, the dapivirine-maraviroc ring. IPM worked with the Oak Crest Institute of Science in 2015 to develop new prototypes that would release higher levels of maraviroc, which IPM acquired in 2008 under a royalty-free license with ViiV Healthcare.
While these prototype rings could inform future combination ARV product development, findings from IPM’s research on maraviroc suggest that its efficacy as a microbicide may be limited. IPM, along with its Scientific Advisory Board and Board of Directors, has suspended development of maraviroc, in keeping with IPM’s prioritization protocol to put resources behind the ARVs that show the highest likelihood of success as vaginal microbicides. IPM will reassess maraviroc’s potential as a microbicide when additional data from ongoing clinical trials of maraviroc are available.
One promising compound now prioritized in IPM’s pipeline is darunavir, a potent protease inhibitor currently used for HIV treatment. In 2015, IPM received an exclusive, royalty-free license for darunavir from Janssen Sciences Ireland UC, one of the Johnson & Johnson Pharmaceutical Companies. Next steps include characterization studies to assess the drug’s properties and prototype development of a darunavir-based combination ARV ring.

Supporting vaginal and rectal gels
A number of vaginal and rectal gels are being studied by our clinical trial partners. In 2015, IPM supplied the clinical materials for a Phase I study of a dapivirine-darunavir vaginal gel conducted by the European Commission-funded CHAARM program, and for the University of Pittsburgh-led CHARM-03, a Phase I study of a maraviroc- based rectal gel. IPM also collaborated with MTN to design two Phase I studies of dapivirine rectal gel expected to begin in 2017.



Strengthening the Body of Evidence for Women’s HIV Prevention
IPM contributed to the HIV prevention research base with presentations and publications in major scientific venues in 2015. Highlights included presenting data from supporting studies of the dapivirine ring at the 2015 Conference for Retroviruses and Opportunistic Infections in Seattle and on the dapivirine-contraceptive ring at the 2015 Controlled Release Society Annual Meeting in Edinburgh. IPM published work on the multipurpose ring and the dapivirine-maraviroc ring and gel in leading peer-reviewed journals, including the Journal of Acquired Immune Deficiency Syndromes, Current Opinion in HIV and AIDS, Current Obstetrics and Gynecology Reports, Pharmaceutical Research and Advanced Drug Delivery Reviews.


Above: Ring Study research center staff at an adherence event.
IPM Donors
2015 Donors
Bill & Melinda Gates Foundation
Flanders Department of Foreign Affairs
Irish Aid, Department of Foreign Affairs
Ministry of Foreign Affairs of Denmark
Ministry of Foreign Affairs of the
Netherlands through the Netherlands
Enterprise Agency
Norwegian Agency for Development
Cooperation, Norwegian Ministry of
Foreign Affairs
United Kingdom Department for
International Development
United States Agency for International Development through the United States President’s Emergency Plan for AIDS Relief
Previous Donors
Ackerman Family Foundation
Belgian Development Cooperation
Canadian International Development Agency
European Commission
Federal Ministry for Economic
Cooperation and Development, Germany
M•A•C AIDS Fund
Magee-Womens Research Institute and
Foundation
Ministry for Foreign Affairs, Sweden
Ministry of Foreign Affairs and Cooperation, Spain
Ministry of Foreign Affairs, France
OPEC Fund for International Development, the development finance institution of OPEC Member States
Rockefeller Foundation
Swedish International Development Agency
United Nations Population Fund
World Bank
Board of Directors
James McIntyre, MBChB, Chair
Anova Health Institute, South Africa
Ayo Ajayi, MD, MPH
Bill & Melinda Gates Foundation, Ethiopia
Pamela W. Barnes, MBA
Independent Consultant, United States
Bruce Burlington, MD
Independent Consultant, United States
Georgina Caswell, MA
International HIV/AIDS Alliance, South Africa
Tamar Howson, MS, MBA
Independent Consultant, United States
Florence W. Manguyu, M.Med, MBChB
Aga Khan University Hospital; International AIDS Vaccine Initiative, Kenya
David Nicholson, PhD
Allergan, United States
Ndola Prata, MD, MSc
University of California, Berkeley School of Public Health, United States
Zeda F. Rosenberg, ScD
IPM, United States
Michael Stevens
ENTHUSE Charitable Trust, United Kingdom
Heidemarie Wieczorek-Zeul
Former Member of Parliament, German Bundestag, Germany
Scientific Advisory Board
Robin Shattock, PhD, Chair
Imperial College London, United Kingdom
David R. Friend, PhD
Evofem, United States
Sharon Hillier, PhD
Magee-Womens Hospital, University of Pittsburgh School of Medicine, United States
Ruth B. Merkatz, PhD, RN, FAAN
Population Council, United States
Thomas Moench, MD
ReProtect, Inc., United States
Derek Newall, PhD
Independent Consultant, United Kingdom
Doug Taylor, PhD
FHI 360, United States
Jim A. Turpin, PhD
National Institute of Allergy and Infectious
Diseases, National Institutes of Health,
United States
Lut Van Damme, PhD, MD
Bill & Melinda Gates Foundation, United States
Jens Van Roey, MD
Janssen Infectious Diseases - Diagnostics BBVA, Belgium
IPM Leadership
Zeda F. Rosenberg, ScD
Founder and Chief Executive Officer
Brid Devlin, PhD
Executive Vice President, Product Development
Kathy Flynn, MBA
Chief Financial Officer
Mike Goldrich, MBA
President and Chief Operating Officer
Patricia R. Mayer, PhD
Executive Vice President, Regulatory Affairs
Annalene Nel, MD, PhD
Executive Vice President, Chief Medical Officer, Clinical Programs
2015 Financial Considerations
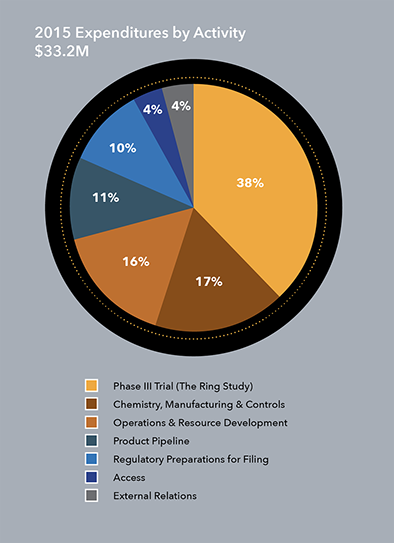
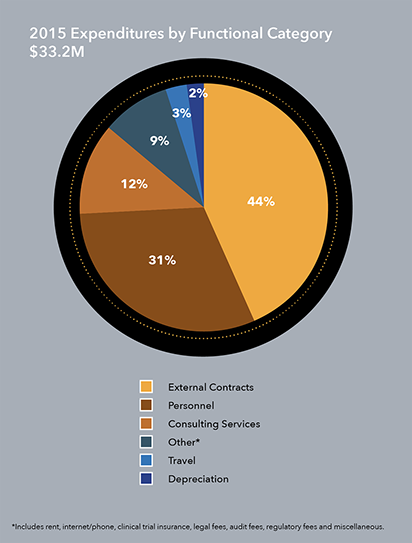
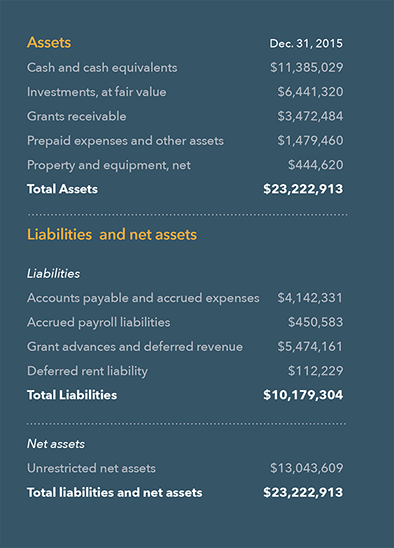
IPM’s cash, cash equivalents and short-term investments as of Dec. 31, 2015, totaled $17.8 million. During 2015, IPM continued work in three major activity areas:
- Support to clinical research centers and clinical activities related to The Ring Study, one of two parallel Phase III clinical trials of the dapivirine ring in Africa. IPM also spent funds to support chemistry, manufacturing and control activities in preparation for a potential application for the ring’s licensure and pre-launch manufacturing requirements.
- Increased regulatory activities to minimize delays in submitting applications for the ring’s licensure once efficacy results were known.
- Pipeline products, including funding for the first clinical trial of DS003, which began in Belgium. Other preclinical work under way includes development of combination ARV rings and a dapivirine-contraceptive ring that would prevent both HIV and unintended pregnancy. This work is conducted using a highly disciplined approach to product prioritization that advances only the most promising self-initiated HIV prevention tools and other sexual and reproductive health technologies for women, and in consultation with IPM’s Scientific Advisory Board.
IPM received significant support from donors including the Ministry of Foreign Affairs of Denmark, the Flanders Department of Foreign Affairs, Irish Aid, the Ministry of Foreign Affairs of the Netherlands, Norad, DFID, USAID and the Bill & Melinda Gates Foundation. IPM received approximately USD $25.1 million (cash receipts) in 2015.
IPM’s 2015 financial audits continue a history of full compliance with all financial reporting requirements from all domestic and international government and private donors. In 2015, IPM again received unqualified, or clean, opinions on all audits in both its South Africa and US offices.
IPM’s Board of Directors, management team and staff are committed to efficiently and effectively delivering on our mission to advance new methods to protect women around the world from HIV infection. Sustained donor funding for IPM’s success is essential, and we continue to advocate for increased funds from existing donors and to pursue new sources of support to achieve our goals.